Detection of SARS-CoV-2 IgM/IgG
NUEVO COVID-19 IgM/IgG Kit is a chromatographic immunoassay kit for rapid, qualitative and convenient detection of SARS-CoV-2 antibodies in human serum, plasma or whole blood. This test is for in vitro professional diagnostic use of patient with clinical symptoms with SARS-CoV-2 infection.
The nitrocellulose membrane is coated with two test lines (G and M) and a control line. Both the control line and test lines in the result window are not visible before applying any specimens. Monoclonal anti-COVID-19 antibody is coated on the control line region and Monoclonal anti-human IgG is coated on the test line region in IgG device and Monoclonal anti-human IgM is coated on the test line region in IgM device. And also, human IgM/IgG-specific antibody is conjugated to the colloidal gold particles. This conjugate is placed on a polyester or glass fiber as conjugate pad. When the sample is dropped into the sample well on the device, the solubilized conjugate migrates with the sample by passive diffusion and both the conjugate and sample come into contact with the antibody that immobilized onto the nitrocellulose. If the sample contains COVID-19 IgM/IgG antibodies, the result is visible as red line within ~10 minutes in the test line on the membrane. The solution continues to migrate to encounter a control reagent that binds a control conjugate, thereby producing another red control line.
Product: NUEVO COVID-19 IgM/IgG
Category: Diagnosis Kit
Date: March 2021
Component

Test Process
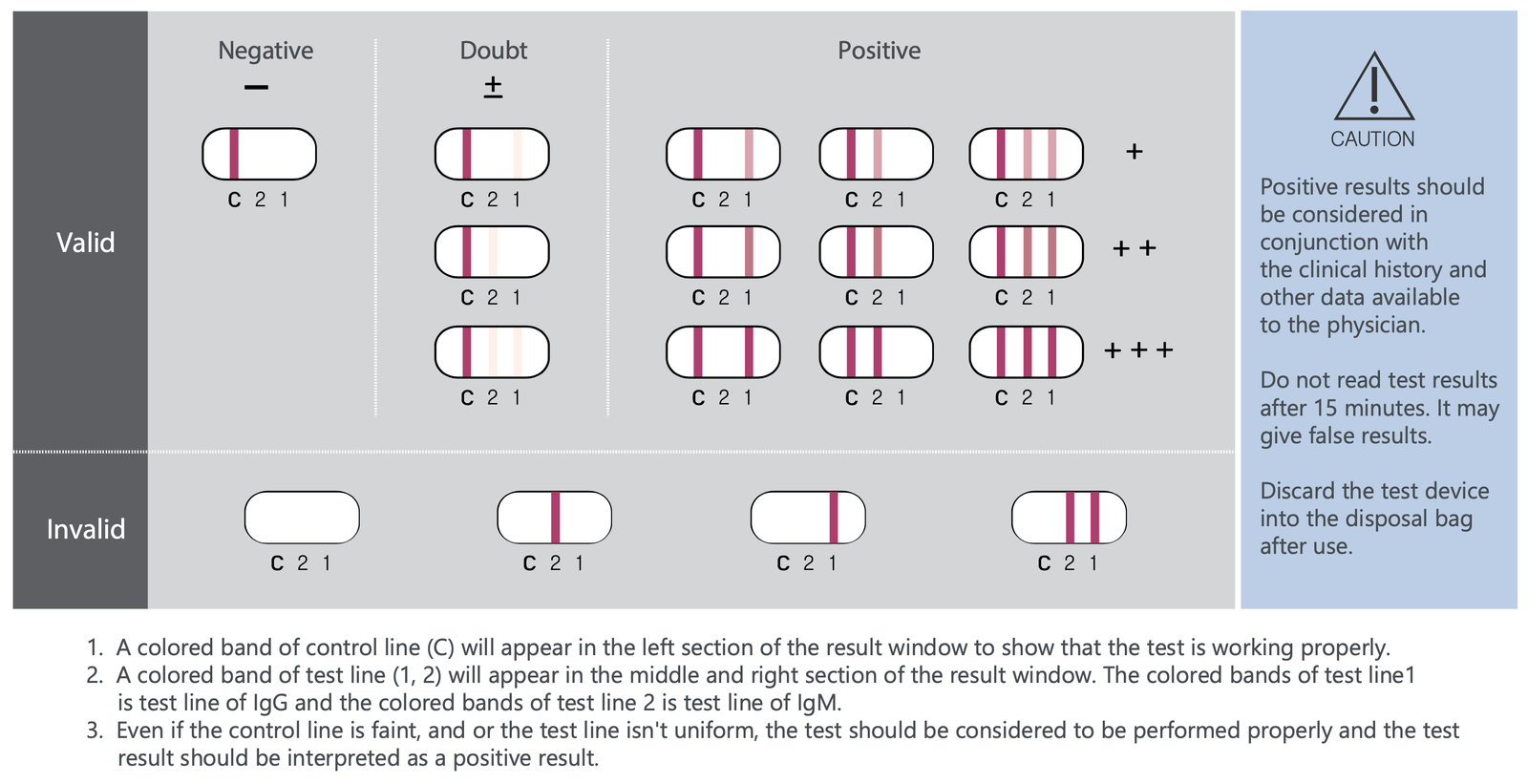
Interpretation of test result
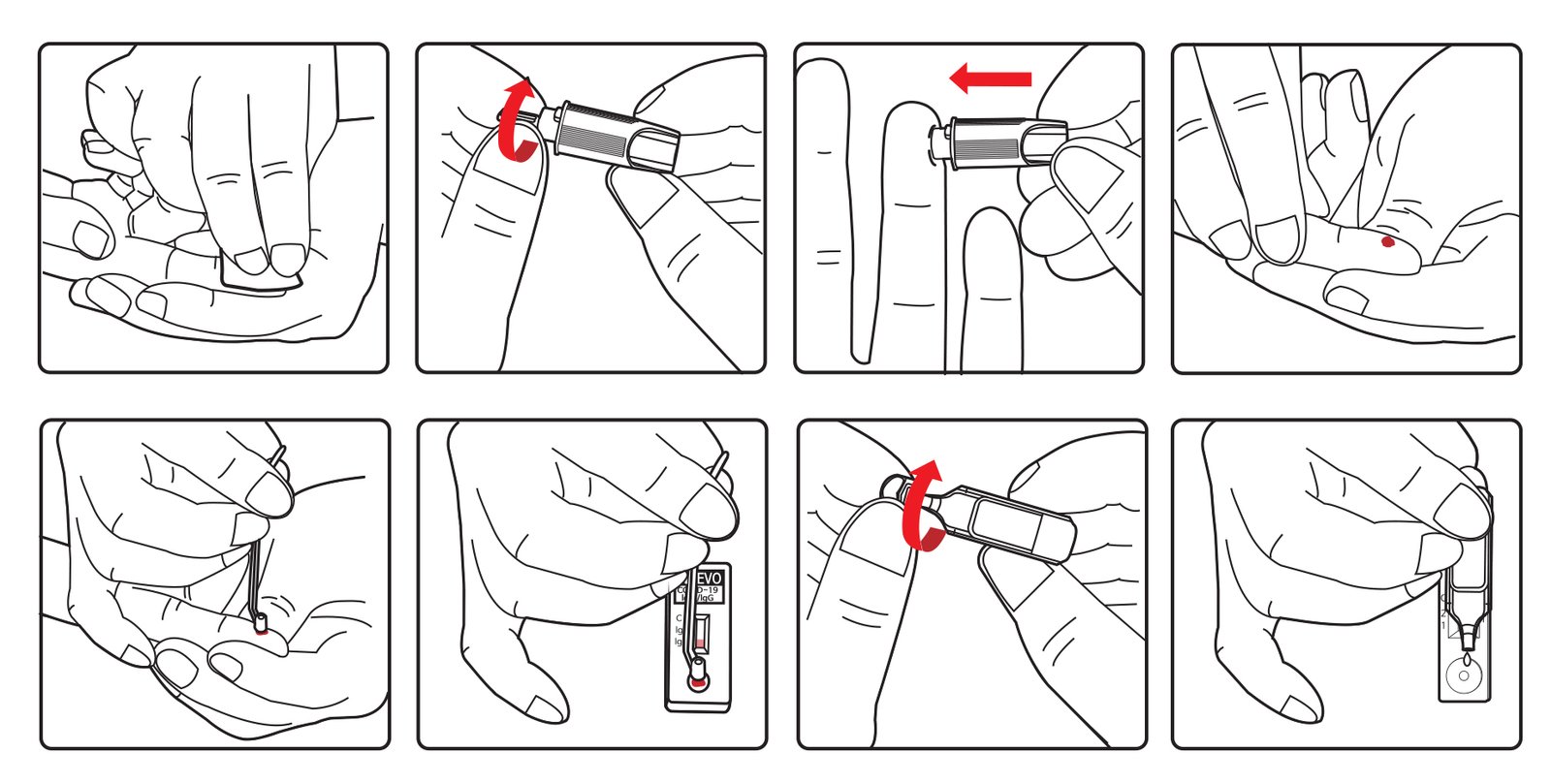
Instruction for Use
- NUEVO COVID-19 IgM/IgG IFU English version [download]


